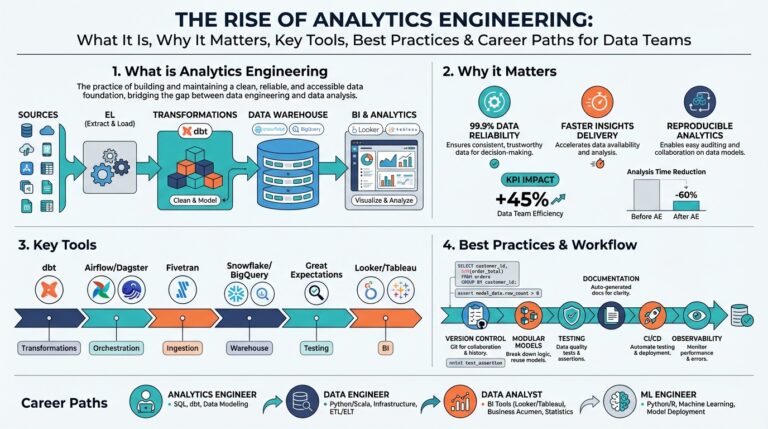
The Intersection of Language Models and Life Sciences
The convergence of advanced language models and biological research is opening new avenues for scientific exploration, making the once-impenetrable language of life increasingly accessible to both researchers and machines. At the heart of this transformation are large language models (LLMs) like GPT-4 and Google’s MedPaLM, which are being trained on enormous datasets comprised not just of human language, but of biological texts, genomic data, protein sequences, and clinical literature. This fusion of computational linguistics and life sciences is generating unprecedented insights and accelerating the pace of discovery across multiple disciplines.
Deciphering Biological Data at Scale
Biological data, from genetics to proteomics, is inherently complex and unstructured. Traditional computational methods often required strict data formats and predefined queries. In contrast, LLMs excel at parsing, interpreting, and generating natural language—including the technical jargon, shorthand, and nuanced context found in scientific literature. These models can read through thousands of academic papers, interpret gene sequences, or summarize trends in genomics faster than any human. Researchers from Nature have highlighted how tools powered by LLMs are already scouring databases, annotating genomes, and identifying potential drug targets, vastly reducing the time required for such analyses.
Creating New Scientific Knowledge
Language models are not only consumers of knowledge—they are becoming co-creators. By training on vast corpora of life science literature, models can detect patterns or propose hypotheses that might not be obvious even to domain experts. For example, the OpenAI GPT family has been used to generate novel protein sequences with desirable properties or to predict the impact of genetic mutations on protein function. Step-by-step, a researcher can input raw sequence data, prompt the model for potential modifications, and receive predictions or rationales based on hundreds of thousands of analogies found in the literature, as described by a study in Cell.
Bridging Communication Gaps
The life sciences encompass vast areas of expertise, each with its jargon and conventions. LLMs act as ‘translators’—able to convert clinical data or dense research findings into plain language or vice versa. This democratizes knowledge by making cutting-edge research more understandable to interdisciplinary teams, policy makers, and even patients. Academic platforms like Science Daily have covered how these tools are being used to summarize long research articles, helping non-specialists grasp the significance of breakthroughs in areas like synthetic biology or personalized medicine.
Enabling Novel Experimental Approaches
At the intersection of language models and laboratory science, entirely new research methodologies are emerging. For instance, LLMs can suggest experimental designs or optimize protocols by referencing millions of prior studies. In a practical workflow, a biologist might describe their research question and materials to an LLM-based assistant, which then recommends the most successful experimental strategies or highlights potential pitfalls based on previous scientific outputs. The journal Nature discusses AI-powered lab assistants employed in drug discovery and gene editing, underscoring the growing role of LLMs as collaborators, not just tools.
As language models continue learning the intricate dialects of biology, their ability to find meaning in this complexity is transforming how scientists pose questions and solve problems. This ongoing dialogue between artificial and biological intelligence is setting the stage for a new era of discovery at the very core of the life sciences.
Decoding the Language of DNA: How LLMs Interpret Genetic Information
Large Language Models (LLMs) have emerged as powerful tools in decoding the intricate language embedded within DNA. Traditionally, interpreting genetic information required laborious experiments and meticulous analysis by experts. Today, advanced LLMs are expediting these processes, uncovering hidden patterns, and enabling scientists to analyze vast genetic datasets with unprecedented speed and accuracy.
At the heart of DNA lies a sequence composed of four basic nucleotides—adenine (A), thymine (T), cytosine (C), and guanine (G). These sequences act like biological sentences, encoding the instructions for life. The challenge, however, is that the biological “language” of genes is highly complex and context-dependent. Using approaches similar to those used to understand and generate human languages, LLMs are now able to interpret this code with surprising sophistication.
Parsing Genetic Sequences like Natural Language
Much like how LLMs learn to predict the next word in a sentence, they now predict the likelihood and impact of mutations in a sequence of DNA. By being trained on massive genetic databases—such as those made accessible by the National Center for Biotechnology Information (NCBI) GenBank—these models learn patterns and correlations that would be nearly impossible to spot unaided. For example, they can identify regulatory elements, protein-coding regions, or potential disease-causing variants from raw sequence data.
Researchers have applied LLMs to tasks ranging from genome annotation to predicting the structure and function of proteins. For example, the breakthrough work by DeepMind’s AlphaFold—a neural network that accurately predicts protein structure based on genetic sequences—demonstrates how linguistic models can be adapted for biological purposes.
Context Matters: The Role of Sequence Context in Genetic Interpretation
One major advantage of LLMs is their ability to consider the surrounding sequence, much like understanding words in context. Where older algorithms might analyze a mutation in isolation, LLMs evaluate how that variant interacts with its neighbors and the larger genomic environment. This contextual awareness is critical because the effect of a mutation can vary dramatically depending on where it occurs and what else surrounds it.
This ability facilitates the discovery of genetic variants implicated in complex diseases. For example, recent studies published by research institutions like Broad Institute leverage LLMs to map whole-genome data in rare diseases, identifying signals that would be lost in static, rule-based approaches.
Applications: Real-World Impact and Novel Approaches
One of the most exciting applications is the acceleration of personalized medicine. By sifting through the vast expanse of genomic data, LLMs can identify genetic risk factors unique to individual patients, helping clinicians devise tailored therapies. Additionally, LLMs are proving invaluable in synthetic biology, where they suggest optimal ways to design new genes or reprogram existing ones for therapeutic uses.
For instance, projects such as European Bioinformatics Institute services utilize LLMs to automate genome annotation, vastly reducing the manual labor once required. Similarly, in drug discovery, these models rapidly scan genetic profiles to predict how individuals might respond to experimental treatments—a process that typically took years and is now achievable in months or days (see: Nature Biotechnology).
As LLMs continue to evolve, their integration into biological research is poised to unlock novel insights and accelerate discoveries. By learning to “speak” the language of DNA, these models are transforming genetics from a dark art into a science illuminated by data-driven prediction and deep contextual understanding.
From Proteins to Pathways: LLMs in Biological Data Analysis
Recent advances in large language models (LLMs) have transformed biological data analysis. By leveraging their ability to “understand” and generate human-like language, these sophisticated AI systems are making sense of vast, complex biological data sets in ways that were previously unimaginable.
Unraveling Protein Sequences with LLMs
Proteins are the functional engines of life, formed from long chains of amino acids. Traditionally, deciphering the roles and structures of these molecules required laborious lab experiments and specialized software. However, LLMs trained on enormous protein databases now analyze protein sequences, predict structures, and infer functions using natural language representations.
For instance, by treating amino acid sequences like words in a sentence, models can suggest possible 3D shapes a protein may fold into. One high-profile example is AlphaFold, which leverages transformer-based architectures (similar to LLMs used in text generation) to predict protein structures at scale. This capacity accelerates drug discovery, understanding disease mutations, and designing new enzymes for industry.
From Genomes to Pathways: Making Sense of Biological Networks
Biological systems are intricate webs of genes, proteins, and biochemical reactions. Mapping these pathways, such as signaling cascades or metabolic routes, has historically involved piecing together fragmented datasets. LLMs help by integrating diverse biological data—genomes, transcripts, metabolites—and automatically suggesting functional connections and pathway hypotheses.
- Step 1: Data Integration — LLMs parse thousands of scientific papers, extracting relevant relationships between biological entities. They structure this unorganized knowledge to inform pathway modeling.
- Step 2: Hypothesis Generation — By connecting dots within and between datasets, models can propose novel interactions, or suggest missing steps in known pathways for wet-lab validation.
- Step 3: Interactive Exploration — Scientists can query these models conversationally to explore “what-if” scenarios or to get summaries of current biological knowledge about a given process. MIT’s EVEscape LLM-powered assistant is an example: it helps researchers navigate bioscience literature in real time.
LLMs Pushing the Boundaries of Systems Biology
Systems biology seeks to understand how molecular components cooperate to produce life. Here, LLMs serve as both compasses and companions—they scan massive omics databases and generate machine-readable models of how an entire cell or organism might respond to perturbations.
For example, researchers can task models with simulating the effect of a genetic variant on metabolic flux, or ask LLMs to interpret patient multi-omics data to guide personalized medicine. This can guide experiments toward the most promising hypotheses, reducing costs and time.
The ability of LLMs to “speak” biology doesn’t just help biologists work faster—it enables them to ask entirely new kinds of questions. With their prowess in understanding both language and data, these AI models are poised to reveal hidden patterns in the biological universe, pushing science forward in ways we are just beginning to explore.
Training Artificial Intelligence to Understand Scientific Jargon and Context
Training language models to understand the complex world of biology begins with teaching them to recognize, parse, and interpret scientific jargon with contextual precision. Traditional natural language processing (NLP) systems often falter when faced with domain-specific terminology, acronyms, and nuanced phrasing common in biological research. To overcome this, researchers must curate massive, field-specific datasets to expose AI models to the language of biology in its native context.
One crucial step in this process involves assembling corpora from diverse scientific publications, preprints, and biomedical databases. By feeding language models with resources like PubMed Central and arXiv’s quantitative biology category, the AI system encounters real-world examples of scientific writing, ranging from experimental studies to systematic reviews. These texts contain both common and highly specialized vocabulary, enabling the model to associate terms with their proper meanings and contexts. For instance, words like “expression” or “transformation” hold specific, context-dependent meanings in biology that differ substantially from their general English usage—a distinction LLMs must learn to make.
Fine-tuning is another pivotal component. This process involves taking a pre-trained language model—already adept at general language understanding—and further training it on biological context. Researchers use supervision from expert annotators or automated pipelines that flag biological entities, gene names, or species to help teach the model which words are important and how they relate. This targeted learning has resulted in specialized LLMs such as BioBERT and SciBERT, which are renowned for their proficiency with scientific texts and have demonstrated significant improvements in tasks like entity recognition, relationship extraction, and literature-based discovery.
Contextual learning is another area where recent advances shine. LLMs are now able to leverage attention mechanisms that allow them to focus on relevant sections of text, learning subtle patterns in how scientific terms are used. For example, when reading a description of a protein interaction, modern AI doesn’t just scan for keyword matches but evaluates the relationships within the sentence or across several paragraphs, mimicking how a human scientist unpacks dense technical information. This capability is bolstered by advanced modeling techniques like transformer architectures, which you can learn more about in this Google AI Blog post on transformers.
The impact of these advancements is tangible. AI tools are increasingly adept at tasks that require deep understanding, such as automatically generating summaries of complicated biological studies, identifying novel research connections, or suggesting hypotheses based on literature mining. By learning not just to process but to genuinely “speak” the language of science, LLMs are empowering researchers to approach complex problems in ways that would have previously required painstaking human effort and domain expertise. As AI continues to evolve, the seamless integration of contextual language understanding will further accelerate scientific discovery and interdisciplinary collaboration.
Accelerating Drug Discovery: Novel Applications of LLMs in Biotechnology
The integration of Large Language Models (LLMs) into biotechnology is transforming the way scientists approach the complex process of drug discovery. Unlike previous computational tools, LLMs offer unprecedented capabilities in understanding, interpreting, and generating biological data — bridging the gap between raw information and actionable insights.
Revolutionizing Molecular Design and Screening
Traditionally, designing new drug molecules required labor-intensive experiments and incremental modification of known compounds. Now, LLMs trained on massive datasets of chemical structures and biological interactions can generate novel drug candidates, predict their properties, and suggest synthetic pathways. For instance, research driven by projects like DeepMind’s AlphaFold demonstrates how AI models can predict protein structures, which is crucial for drug targeting. LLMs expand on this approach by hypothesizing about possible adjustments and modifications — helping researchers prioritize molecules with the highest chances of success before stepping into the lab.
- Example: Pharmaceutical companies use LLM-driven platforms such as BenevolentAI to mine scientific literature, clinical trial records, and compound libraries to find novel drug-target interactions, enabling faster candidate identification.
- Step-by-step workflow:
- LLMs process structured and unstructured biological data.
- The models generate molecular suggestions and predict target affinities.
- Scientists validate top candidates experimentally, greatly reducing the number of trial-and-error cycles required.
Enhancing Biomarker Discovery and Target Validation
One of the critical steps in drug development is identifying reliable biomarkers and validating biological targets. Recent advances allow LLMs to synthesize information from genomics, proteomics, and cross-species studies. By analyzing vast swathes of biological literature, patient records, and experimental data, LLMs can uncover previously overlooked connections, predict off-target effects, and recommend personalized intervention strategies.
- Nature Biotechnology highlights the use of LLMs for biomarker identification in oncology, showcasing AI’s ability to recognize disease signatures in big datasets.
- Example: LLM-enabled platforms aggregate patient data from multiple clinical studies, filter noise, and isolate novel biomarker patterns that correlate with positive therapeutic outcomes.
Streamlining Drug Repurposing and Clinical Predictions
LLMs are also reshaping the landscape of drug repurposing—finding new uses for existing medications. With their ability to rapidly scan and interpret millions of clinical notes, scientific abstracts, and adverse event reports, these models help identify promising candidates that can be quickly transitioned to clinical trials, improving the odds of discovering effective treatments for emerging diseases.
- Step-by-step example:
- An LLM scans medical literature and case reports for patterns associated with disease progression and drug response.
- The model identifies medicines with similar interaction profiles or side effects.
- Researchers test these predictions in silico, then validate promising candidates in preclinical or observational studies.
- Initiatives like the NIH-funded National COVID Cohort Collaborative (N3C) have used AI models to highlight existing drugs that might help control COVID-19 progression.
Collaborative Intelligence: LLMs as Partners in Research
The ultimate promise of LLMs in biotech is the emergence of true “collaborative intelligence.” LLMs are not just passive databases but active partners, proposing novel hypotheses, designing virtual experiments, and helping scientists ask better questions. With natural language interfaces, researchers from diverse backgrounds can interact with LLMs, accelerating cross-disciplinary collaboration without needing advanced coding skills. This inclusive approach enhances creativity and enables a broader community of innovators to partake in drug discovery, as discussed by Google AI’s research blog.
As LLMs continue to evolve, they’re set to become an indispensable accelerant in the drug discovery process — not just speeding up research, but fundamentally changing how scientists engage with biological complexity and innovation.
Enabling Multidisciplinary Collaboration Through Natural Language Processing
Scientists have traditionally faced hurdles when working across different disciplines—what might be intuitive language for a computational linguist could be perplexing jargon to a molecular biologist. Large Language Models (LLMs) are rapidly transforming this landscape by leveraging natural language processing (NLP) to make biological data more accessible, understandable, and actionable across diverse scientific domains.
Overcoming the Language Gap Between Disciplines
The specialized vocabulary within biology has often posed barriers to collaboration. LLMs trained on vast and diverse datasets—including research papers, lab reports, and medical records—can act as translators among varied scientific communities. For instance, a cell biologist and a machine learning expert can leverage LLMs to convert complex biomedical concepts into plain language or even schematics, making the content clear and accessible to collaborators from different backgrounds. This paves the way for fruitful multidisciplinary teamwork, allowing for deeper insights and shared progress.
Generating Novel Hypotheses Through Multidisciplinary Data Integration
One of the key strengths of LLMs is their ability to parse and synthesize information from millions of scientific papers and datasets. By seamlessly bringing together linguistic knowledge from genomics, chemistry, medicine, and even computer science, LLMs facilitate the creation of interdisciplinary research hypotheses that might never emerge within a single field. For example, the NIH has reported how AI is helping teams uncover connections in biology by learning from seemingly unrelated disciplines, such as using language models to analyze protein sequences similarly to how sentences are parsed in NLP.
Automating Knowledge Extraction and Summarization
Manual review of the ever-growing biological literature can be overwhelming even for experts. LLMs can be employed to automatically extract relevant findings, summarize recent breakthroughs, and provide tailored literature reviews for teams working at the intersection of biology, mathematics, and engineering. This not only accelerates the discovery process but also democratizes the knowledge by making it instantly available to a broader set of contributors. Recent advancements highlighted by Harvard Data Science Review demonstrate that NLP-powered tools can condense complex research into actionable, readable insights for multidisciplinary teams.
Streamlining Communication and Project Coordination
Collaboration across scientific domains involves frequent communication, data sharing, and consensus building. LLMs can generate emails, meeting notes, or even project plans that accurately reflect technical input from all disciplines involved, ensuring nothing gets lost in translation. Tools like these have already shown promise in biotech startups that blend expertise from software engineering, biology, and clinical research, enhancing their ability to innovate rapidly and cohesively, as discussed by Forbes.
By enabling seamless translation of ideas and facilitating collaboration, LLMs are becoming indispensable not just as data processors but as bridges between the many languages of science. Their evolution promises to unlock unprecedented synergy in the pursuit of understanding life at its most fundamental levels.
Automating Hypothesis Generation: LLMs as Research Partners
The rise of large language models (LLMs) in biological research is transforming the way scientists explore the complexities of living systems. Among the most exciting developments is the ability of LLMs to automate hypothesis generation—one of the most fundamental, creative, and time-consuming steps in the scientific method. Traditionally, hypothesis generation relied heavily on a researcher’s experience, creativity, and ability to synthesize information across vast amounts of scientific literature. LLMs, however, are demonstrating the capacity to support and even accelerate this process, acting as research partners and opening new frontiers in discovery.
Step-by-Step: How LLMs Automate Hypothesis Generation
- Comprehensive Literature Analysis: LLMs can instantaneously assimilate and analyze a corpus of scientific papers, datasets, and experimental results far beyond the capacity of any human researcher. By drawing on resources like PubMed and Nature, these models sift through millions of articles, identifying patterns, gaps, and emerging trends. This extensive, automated review forms the bedrock for suggesting new, unexplored scientific questions.
- Pattern Recognition and Relationship Mapping: Using statistical and computational techniques, LLMs can identify subtle correlations and associations—between genes, proteins, pathways, or clinical outcomes—that might be overlooked by human eyes. For example, recent work at DeepMind has used AI-driven models to predict protein structures, indirectly suggesting new avenues for investigating disease mechanisms and drug targets.
- Translating Natural Language into Testable Propositions: These models excel at “speaking biology”—parsing biological jargon and distilling complex concepts into plain language. Researchers can converse with LLMs in natural language, pose high-level questions, and receive suggestions for testable hypotheses complete with experimental approaches. For instance, scientists working with generative models published by EMBL-EBI have used LLMs to explore connections between metabolic pathways and rare genetic disorders, accelerating the ideation-to-experiment cycle.
- Generating Hypotheses Based on Multimodal Data: As LLMs evolve, incorporating not just text but also data from genomics, proteomics, and clinical imaging, they can propose hypotheses that synthesize disparate forms of evidence. Current research highlighted by Nature shows how LLMs integrate textual knowledge with biological datasets to uncover relationships between cell types and disease progression.
Examples of LLMs as Research Partners
At institutions like The Allen Institute for Brain Science, scientists are working alongside LLMs to suggest experiments ranging from novel gene knockouts in mouse models to alternative strategies for neural imaging. In the pharmaceutical sector, companies are leveraging LLMs to propose new combinations of drug molecules based on previously unconnected literature findings, expediting the early phases of drug discovery.
Crucially, LLMs don’t replace the human element in biological discovery—they amplify it. By relieving researchers of the burden of exhaustive literature reviews and enabling them to focus on creative and critical thinking, LLMs foster a more dynamic and productive research environment. For more on the integration of AI in biology, read the in-depth report from Science Magazine.
As these models continue to evolve, their ability to autonomously generate, refine, and prioritize hypotheses will only grow, marking a paradigm shift in the way science is conducted and accelerating our journey into the unknown corners of biology.
Tackling Big Data: How AI Makes Sense of Complex Biological Systems
The advent of large language models (LLMs) like GPT-4, BioBERT, and other domain-specific AIs has revolutionized the way researchers tackle the overwhelming complexity inherent in biological data. Modern biological research generates massive datasets—from genomic sequences to multi-omics profiling and intricate cellular signaling networks. Making sense of these sprawling data landscapes has become one of the defining challenges of the field. Here, LLMs are acting as powerful assistants, enabling new perspectives and accelerating scientific breakthroughs.
Decoding Genomic Data at Unprecedented Scale
Biology has entered the era of big data due to advancements in next-generation sequencing technologies. Analyzing terabytes of genomic, transcriptomic, or proteomic data requires not just computational power but the ability to interpret subtle biological signals amidst noise. LLMs, trained on vast repositories of scientific literature, clinical data, and annotated sequences, can identify patterns and predict gene function, variant impacts, or regulatory motifs.
- They automate annotation of newly-sequenced genomes, drastically reducing manual labor and time.
- LLMs can hypothesize functional elements in non-coding DNA, as seen in the work published by NIH researchers analyzing genetic variants with AI.
Unlocking Complex Biological Networks
Traditional computational biology tools often fall short when it comes to modeling dynamic, high-dimensional systems such as protein interactions or cellular signaling pathways. LLMs can synthesize information from thousands of papers and datasets to propose new models, spot inconsistencies, or predict the impact of unknown interactions.
- Network Deconvolution: By training on experimental interactome data, LLMs can suggest novel protein-protein or gene-regulatory relationships, an approach explored in recent Cell papers on disease mechanisms.
- Drug Discovery: AI models like AlphaFold have predicted protein structures for nearly every known protein, transforming drug discovery by modeling how compounds interact with targets (Nature News).
Bridging Human Expertise and Machine Insight
LLMs extend researchers’ capabilities beyond any single individual’s knowledge. A scientist can now query an LLM about potential biomarkers, optimal experimental protocols, or connections between disparate datasets, receiving synthesized answers rooted in the collective wisdom of the scientific community. This democratizes access to highly specialized insights, enabling biologists—regardless of their computational background—to exploit big data.
- For instance, the Human BioMolecular Atlas Program (HuBMAP) leverages AI to map the human body at single-cell resolution, with LLMs helping integrate spatial, transcriptomic, and clinical data for holistic biological understanding.
- AI-powered platforms surface connections that might take human experts years to discover, accelerating the translation of bench discoveries into clinical interventions.
As biological data continues to grow in volume and complexity, LLMs serve as indispensable partners—analyzing, predicting, and proposing novel hypotheses. This synergy between artificial intelligence and laboratory science opens the door to discoveries that were once thought impossible.
Ethical Considerations and Challenges of AI in Biological Research
One of the most profound shifts brought by large language models (LLMs) to biology is not just in what they can do, but how they may challenge longstanding norms and raise new ethical questions. As these AI systems become partners in biological discovery, researchers and institutions must grapple with concerns that go far beyond data analysis or modeling. Here are several critical dimensions where ethical considerations and practical challenges intersect, shaping the responsible future of AI in biological research.
Data Privacy and Consent
LLMs are often trained on vast datasets containing sensitive genetic, medical, and personal information. Ensuring the privacy of research subjects—whether patients in clinical studies or contributors to genetic databases—is paramount. Mishandled data can lead to breaches of confidentiality or unintended exposure of identifiable information.
For example, even anonymized genetic data has been shown to be vulnerable to re-identification under certain circumstances (Nature). Ethical practice demands rigorous de-identification, robust encryption, informed consent tailored to AI-driven research, and ongoing assessment of privacy risks. With LLMs that can infer patterns from text, special attention must be paid to the training data’s origin and how it is processed before use.
Algorithmic Bias and Fairness
LLMs, like all machine learning models, can perpetuate and even amplify existing biases present in their training data. In biological research, this can manifest in several ways: overrepresenting certain populations in genetic studies, marginalizing rare diseases, or reinforcing historical health disparities. For example, AI-driven drug discovery tools have sometimes failed to include data from non-European populations, leading to less accurate predictions for diverse patients (NEJM).
Ethical research requires critical evaluation of dataset composition. In practice, this means:
- Conducting bias audits on training data and model outputs
- Seeking diverse and representative data collection
- Employing techniques to mitigate bias in both inputs and predictions
Guidance from organizations such as the National Institutes of Health (NIH) can aid labs as they work to ensure fairness.
Transparency and Explainability
As LLMs generate increasingly complex hypotheses or interpret massive datasets, researchers often face a “black box” dilemma: why did the model suggest a particular interaction or highlight a specific gene? Transparency and explainability are crucial for both scientific rigor and trust in the findings.
Tools such as AI explainability frameworks help scientists trace model outputs to specific data features or rationales. However, these tools are not yet universally adopted, and their effectiveness can vary based on the complexity of the LLM and the nature of the task. A transparent AI system allows biologists to:
- Validate findings against known biology
- Understand failure modes and correct them
- Build trust among interdisciplinary teams and stakeholders
Intellectual Property and Attribution
AI-driven biological research raises questions about who owns discoveries generated through LLM assistance. If an LLM uncovers a novel protein structure or generates a new gene-editing strategy from a database, should the credit go to the researchers, the institution, or even the developers of the AI?
Clear guidelines, as proposed by groups like the World Intellectual Property Organization (WIPO), are needed to ensure fair attribution, protect against inadvertent plagiarism, and encourage responsible, open innovation.
Dual-Use Concerns and Biosecurity
LLMs can accelerate research in vaccine design or synthetic biology, but this dual-use capability also raises the specter of misuse. For instance, AI tools could inadvertently assist in the creation of harmful biological agents if safeguards are not in place (Scientific American).
Researchers and governments alike must develop protocols for responsible usage, including:
- Access controls on sensitive model capabilities
- Ethical training for scientists in dual-use awareness
- Robust collaboration between AI developers, ethicists, and regulatory agencies
As the marriage between LLMs and biology deepens, addressing these ethical and logistical challenges head-on will be key to unlocking responsible, equitable, and groundbreaking scientific advances.